What Best Describes Cytokine Release Syndrome Crs
Synovial sarcoma SS ovarian cancer OC myeloma. CRS has been described after the infusion of several antibody-based therapies including rituximab 10 11 obinutuzumab 12 alemtuzumab 13 brentuximab 14.
Cytokine release syndrome CRS is a CAR-T therapy-related adverse event.
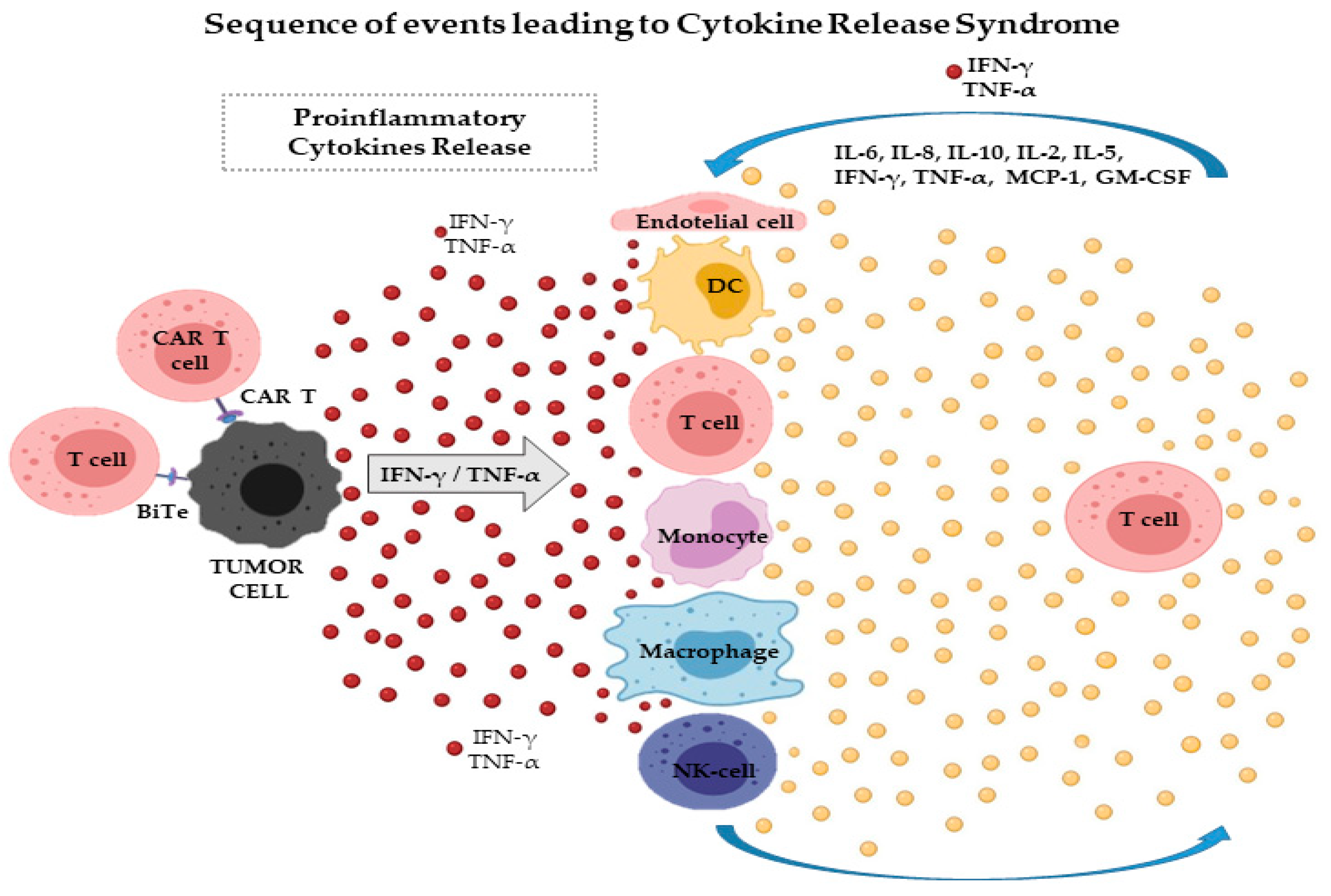
. Cancer Answer Line 8662238100. Cytokine-release syndrome is a symptom complex associated with the use of many monoclonal antibodies. It is also referred as infusion reaction that results in release of large amount cytokines Like IL-6 IFN-r INF from the target cells.
7-8 days Manifestation may include. In this case we observed nivolumab-induced CRS in a patient with gastric cancer. Commonly referred to as an infusion reaction it results from the release of cytokines from cells targeted by the antibody as well as immune effector cells recruited to the area.
Cytokine release syndrome CRS is a collection of symptoms that can develop as a side effect of certain types of immunotherapy especially those which involve T-cells. The physiopathology of CRS is caused by the release of effector cytokines IFNγ TNFα Interleukin 2 which are responsible for activating monocytes and macrophages. Cytokine release syndrome CRS is a life threatening toxicity associated numerous immunotherapeutic techniques involving monoclonal antibodies bispecific antibodies and adoptive T cell therapies.
Bio-Techne offers the most extensive portfolio of immunoassay platforms on the market for measuring CRS-associated inflammatory cytokines. Atrial fibrillation and ventricular tachycardia. We assessed incidence healthcare resource utilization HCRU and costs of CRS and NE in pts in TRANSCEND receiving liso-cel an investigational anti-CD19 CAR T cell product administered as a defined composition of CD4CD8 CAR T cells.
What Is Cytokine Release Syndrome. Cytokine release syndrome CRS is a potentially life-threatening systemic inflammatory response observed following administration of antibodies and adoptive T. Starting as a fever it can rapidly progress to hypotension hypoxia and end-organ dysfunction.
The onset and peak of Cytokine Release Syndrome CRS generally occurs in the first week after CART infusion and precedes the onset of ICANS Immune Effector Cell-associated Neurotoxicity Syndrome. Cytokine release syndrome CRS can cause a variety of symptoms including fever headaches and nausea. Cytokine release syndrome CRS is a systemic inflammatory response that can be triggered by a variety of factors such as infection and certain medications including monoclonal antibodies.
Cytokine release syndrome CRS or cytokine storm is an acute inflammatory syndrome caused by the activation of immune cells and release of proinflammatory cytokines. Among the 7MM countries the United States had the highest market size. Cytokine Release Syndrome CRS.
Grading and management of cytokine release syndrome in patients treated with tisagenlecleucel in the JULIET trial. However the immune activation responsible for high remission rates is also responsible for the unique treatment-related toxicity of cytokine release syndrome CRS. Cytokine release syndrome CRS happens when your immune system responds to infection or immunotherapy drugs more aggressively than it should.
Cytokine release syndrome CRS is an acute systemic inflammatory syndrome characterized by fever and multiple organ dysfunction that is associated with chimeric antigen receptor CAR-T cell therapy therapeutic antibodies and haploidentical allogeneic transplantation. While mild cases can present as flu-like illness more severe responses may lead to of life threatening cardiovascular pulmonary and. Cytokine release syndrome CRS is a potentially life-threatening systemic disease that has been observed after treatment with antibodies and adoptive T cell therapies.
Immune effector cell-associated neurotoxicity syndrome ICANS is a neuropsychiatric syndrome that. Cytokine release syndrome CRS is observed with adoptive T-cell therapies ACT with varying frequency. The clinical signs of CRS include fever hemodynamic instability and capillary leak which correlate with T-cell activation and elevated cytokine levels.
In June 2018 in an effort to standardize CRS grading the American Society for Transplantation and Cellular Therapy ASTCT developed the ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells Tables 2 4. Cytokines are small proteins that act as cell messengers. CRS analyte One of the most prominent toxicities associated with cancer immunotherapies is cytokine-release syndrome CRS which can be fatal if not properly identified and managed.
Fever hypotension tachycardia hypoxia and chills. While CAR-associated Hemophagocytic Lymphohistiocytosis-like syndrome CarHLH manifestations may overlap with CRS it has also been described as a. CRS prevalence and severity within the context NY-ESO-1 C259 T across 5 studies in 4 tumor types were examined.
CRS occurs when the immune system responds too. When cytokines are released into the circulation systemic symptoms such as fever nausea chills. Serious events may include.
CRS symptoms include fever nausea fatigue and body aches. 2-3 days Typical duration. The market size of Cytokine Release Syndrome CRS in the 7MM was found to be USD 016 million in 2017.
The syndrome occurs when immune cells are activated and release large amounts of cytokines into the body. HCRU within the dates of onset and resolution of CRS and NE was identified from case report. CRS can be life threatening and may require steroids which hinder the effectiveness of ACT.
Prompt treatment is essential as symptoms can worsen quickly. These new treatments are associated with rare toxicities such as cytokine release syndrome CRS defined by fever tachypnea headache tachycardia hypotension skin rash andor hypoxia. To date clinical trials of different CAR-T products have not been align.
Cytokine release syndrome CRS is a systemic inflammatory response that may be initiated by a variety of factors including T-cell therapies. Recognize Symptoms Early Typical onset. CRS may be associated with cardiac hepatic andor renal dysfunction.
CRS was defined by the ASTCT consensus group as a supraphysiologic response following any. Cytokine release syndrome CRS is an acute systemic inflammatory syndrome characterized by fever and multiple organ dysfunction that is associated with chimeric antigen receptor CAR-T cell therapy therapeutic antibodies and haploidentical allogeneic transplantation. A 43-year-old male with advanced gastric cancer was treated with nivolumab as a third-line.
The symptoms can become severe quickly.
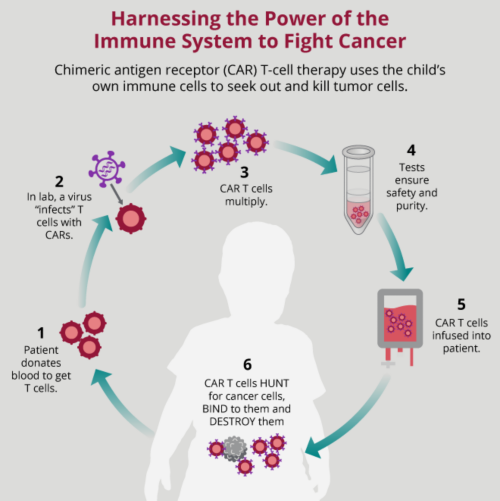
Cytokine Release Syndrome Crs After Immunotherapy Together

Ijms Free Full Text Cytokine Release Syndrome Associated With T Cell Based Therapies For Hematological Malignancies Pathophysiology Clinical Presentation And Treatment Html


Comments
Post a Comment